[Feature] Prof. Yon‘s Research Team Develops Explainable AI for Early Detection of Kidney Disease in Diabetes
A research team led by Professor Yon Dong-keon and Rhee Sang-youl of the Kyung Hee University (KHU) College of Medicine, including Research Professor Woo Se-lin and researchers Cho Jae-hyeong, Hwang Seung-ha, and Kim So-eun, has developed an artificial intelligence (AI) model that predicts the five-year risk of chronic kidney disease (CKD) in patients with type 2 diabetes mellitus (T2DM) using retinal fundus images together with routine clinical data. The findings were published in July 2025 in Diabetes Care as A Multimodal Predictive Model for Chronic Kidney Disease and Its Association With Vascular Complications in Patients With Type 2 Diabetes: Model Development and Validation Study in South Korea and the U.K.

From left: Professor Yon Dong-keon, Research Professor Woo Se-lin, researcher Kim So-eun
Why Multimodal AI Is Needed for Early CKD Risk in T2DM
CKD is a progressive decline in the kidneys’ ability to filter waste and excess fluid—a common complication of diabetes. Without early detection, it can advance to end-stage disease requiring dialysis or transplantation. However, early-stage CKD is difficult to identify. “Especially in aging societies like Korea, there is an urgent need for tools that can predict risk in advance from a preventive perspective,” said Prof. Yon.
However, conventional prediction methods that hinge on single indicators, such as blood glucose, show limited accuracy. Likewise, many AI studies have remained single-modal, relying on a single data type such as clinical records, which reduces predictive performance and hinders explainability.
In response to these challenges, Prof. Yon’s research team sought a more precise and reliable direction for CKD risk prediction. In particular, the team focused on retinal fundus images of patients with T2DM, which reveal diabetic microvascular changes closely linked to kidney health. The researchers reasoned that a multimodal approach integrating fundus images with routinely collected clinical data could improve the accuracy and clinical utility of risk estimates for individual patients.
To put this multimodal direction into practice, the team turned to AI. Prof. Yon noted, “Medical data are highly complex and vary from patient to patient, making it difficult to adequately address using only traditional statistical methods alone. In this context, AI can analyze large-scale medical data, detect hidden patterns that are hard for humans to discern, and enhance predictive performance.” He added that these capabilities underpinned the present study.
Integrating Clinical Data and Fundus Images for CKD Risk
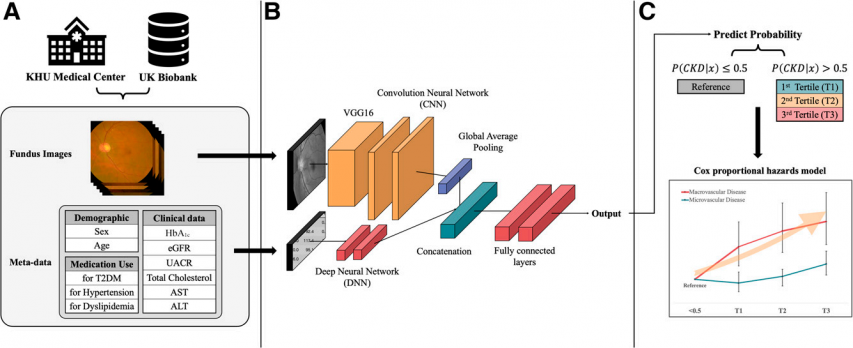
The team trained the model on medical records from 7,028 domestic patients with T2DM treated at KHU Medical Center. They used two inputs: routinely collected clinical data—including blood and urine tests, medication use, medical history—and retinal fundus images. Clinical variables were modeled with a deep neural network (DNN) to estimate five-year CKD risk, while fundus photographs were analyzed with a convolutional neural network (CNN) based on VGG16 to capture subtle retinal microvascular changes.
The DNN and CNN outputs were integrated to generate a single CKD risk estimate for each patient. By combining clinical data and fundus images in a multimodal AI model, the modalities complemented one another, resulting in higher predictive performance than single-modal models.
Model development and testing proceeded stepwise. The domestic cohort was split into training and internal validation sets to train and optimize the model, after which the finalized model underwent external validation in the UK Biobank. Leveraging both Korean and U.K. datasets demonstrated the model’s generalizability across diverse patient populations.
With performance and generalizability established, the team next addressed explainability, a prerequisite for clinical adoption. The research team highlighted explainable AI (XAI) as a key feature of the model. Although AI offers strong predictive performance, its “black box” nature makes it difficult to explain how predictions are derived, which limits use in medicine. “If the basis of AI’s judgment is unclear, it is difficult for medical professionals to trust and apply it in clinical practice. In medicine, where lives are at stake, it is more important to provide a clear explanation of why this patient was classified as high-risk than just the prediction result,” said Prof. Yon.
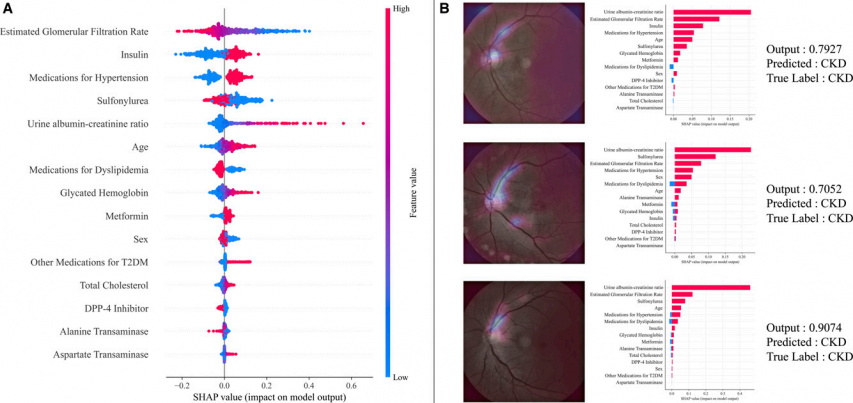
To support clinician trust and understanding, the team applied two explanation techniques. One was Shapley additive explanations (SHAP), which quantifies how much each input contributes to a given prediction. In the SHAP analysis, the strongest contributors to CKD risk included estimated glomerular filtration rate, an indicator of how well the kidneys filter blood, use of antidiabetic and antihypertensive medications, and age.
The second technique, Gradient-weighted Class Activation Mapping (Grad-CAM), visualizes the image regions the model relied on when generating a prediction. Applied to retinal images, Grad-CAM produced heatmaps that highlighted the optic disc and superior arched vessels in patients the model flagged as high CKD risk. By incorporating such XAI techniques, the research team aimed to help medical professionals understand the AI’s decision-making rationale and facilitate its practical use in clinical settings.
From Accuracy to Action: Explainability and Patient Tools
The optimized model showed strong discrimination in validation. In internal validation on the Korean cohort, the five-fold cross-validated area under the receiver operating characteristic curve (AUROC) was 88.0%. In external validation on U.K. patients, AUROC was 72.2%, indicating stable performance across diverse patient groups. In medical AI, AUROC values near or above 80% are generally considered excellent, underscoring the model’s potential for reliable application.
Furthermore, the superiority of the multimodal model was confirmed in comparisons with single-modal approaches. In head-to-head testing, the multimodal model outperformed the image-only model by more than 16%, providing empirical evidence for integrating diverse information sources. In other words, by jointly reflecting clinical information and imaging data, the model demonstrated greater precision in capturing future CKD risk.
Another noteworthy finding is that the model predicts risk beyond kidney outcomes. In follow-up analyses, patients the AI classified as high risk showed higher incidence of diabetic complications. The higher the predicted risk tier, the greater the burden of events, with macrovascular complications such as myocardial infarction and stroke occurring at more than 1.6-fold higher rates and microvascular complications including retinopathy and neuropathy also significantly elevated.
This indicates that the model’s predictions capture a patient’s overall vascular health. Patients with T2DM whom the model flags as high kidney risk therefore require closer management of other complications. Because a single prediction can signal the risk of multiple complications, the model developed by Prof. Yon’s team offers practical value for long-term patient health management and the design of preventive strategies.
Using XAI, the team also prioritized making prediction results understandable not only to healthcare providers but to patients. Their premise was clear: prediction has value only when patients can recognize their own health status and use it to guide prevention and lifestyle improvement.
To this end, the team developed a web-based patient prediction result guide system for real clinical use. Patients enter their information into the system, which then shows their five-year CKD risk, major risk factors, prediction results by model type, and personalized lifestyle improvement recommendations. By making the results easier, this approach positions medical AI not just as a diagnostic aid but as a practical management tool that supports patient behavior change.
The significance of this study lies not only in developing a high-performance predictive model but also in its immediate clinical applicability. The model uses information routinely collected in diabetes care rather than special tests or costly equipment. By entering routine clinical data, kidney disease risk can be estimated without additional burden, making the approach suitable for primary healthcare settings such as local clinics and public health centers. Prof. Yon said, “Considering these characteristics, the model could be applied in primary care institutions to identify high-risk groups early and connect them to higher-level medical institutions.”
Building on this work, the team is broadening prediction targets to other chronic diseases such as cardiovascular disease and Alzheimer’s disease, as well as conditions like Crohn’s disease and hair loss. In the longer term, they aim to develop a multimodal foundation model that integrates genomic information, lifestyle factors, and wearable data to estimate multiple disease risks for each individual.
By integrating two distinct data types, clinical information and retinal images, the study achieved high accuracy and explanatory power, showing that early detection of CKD risk—difficult to achieve with clinical data alone—is feasible. As AI increasingly supports forecasting and prevention in healthcare, this work stands as a leading case of medical AI with significant implications.
However, under current law, AI systems cannot independently provide diagnoses or predictions, and careful attention to personal data protection and data ethics remains essential. To address these constraints, the team incorporated XAI so medical staff can understand the basis of each prediction, and they plan to demonstrate the model’s safety and effectiveness through multi-institutional collaboration and verification in real clinical settings.
Looking ahead, Prof. Yon emphasized the study’s guiding principle. “I believe that medical AI should go beyond mere technological development and contribute to protecting the health of actual patients. In particular, predictive models for diseases with high public health value should be designed fairly and made accessible to everyone. I will continue to work to connect clinical practice and policy through data-driven research,” he said.
As evidence accumulates, early prediction of chronic disease risk—and timely alerts and management—based on simple clinical information and imaging data could become part of routine care. The convergence of technology and healthcare is moving toward practice, with the potential to improve patients’ lives in the near future.
There are no registered comments.
I agree to the collection of personal information. [view]